3-Parent DNA: Preventing Inherited Disease
Mitochondrial disorders are a group of chronic, inherited diseases that can cause a wide array of severe health problems.
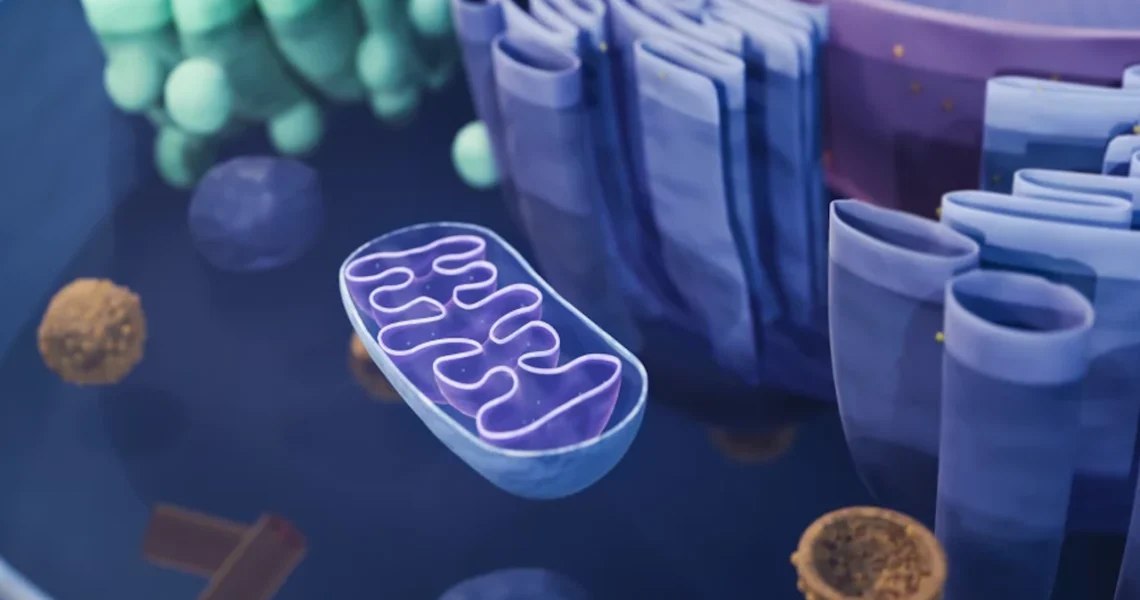
These conditions occur when the mitochondria, often referred to as the “powerhouses” of the cell, fail to produce enough energy for the body to function properly. Mitochondria are responsible for generating over 90% of the energy needed by the body to sustain life and support organ function. When their function is impaired due to genetic defects, the consequences can be devastating.
The symptoms of mitochondrial disorders are incredibly varied and can affect almost any organ system in the body, as every cell relies on mitochondria for energy. Common severe health problems associated with these disorders include:
- Paralysis: Due to muscle weakness and nerve damage.
- Heart failure: The heart muscle is highly energy-dependent.
- Brain damage: Leading to cognitive decline, developmental delays, and seizures.
- Strokes: As a result of impaired blood flow and cellular dysfunction.
- Blindness: Due to optic nerve damage or retinal degeneration.
Children born with one of these severe disorders often endure short, painful lives. The progressive nature of these diseases means that symptoms can worsen over time, leading to significant suffering and a severely reduced quality of life. For families, watching a child slowly succumb to such a debilitating illness is profoundly tragic and heartbreaking.
The root cause of these disorders lies in defects within the mitochondrial DNA (mtDNA). Unlike the vast majority of human DNA, which is found in the cell’s nucleus and inherited from both parents, mtDNA is located exclusively within the mitochondria. Crucially, this genetic material is passed down only from mother to child. This maternal inheritance pattern means that a mother with defective mtDNA, even if she herself shows no symptoms (a carrier), has a high risk of passing the disorder to all her offspring. Addressing these mtDNA defects is the central challenge that the “three-parent” baby technique seeks to overcome.
Mitochondrial Donation: How the “Three-Parent” Technique Works
The groundbreaking solution tested by the British scientists to prevent mitochondrial disorders is technically referred to as “mitochondrial donation” or sometimes “mitochondrial replacement therapy (MRT).” This innovative procedure effectively circumvents the transmission of defective mitochondrial DNA from a mother to her child, while ensuring the child retains the vast majority of genetic material from its biological parents.
The process involves several meticulous steps:
- Preparation of the Parents’ Fertilized Egg: The procedure begins with a fertilized egg from the couple who wishes to have a healthy baby. This egg contains the nuclear DNA from both the biological mother and father, representing the vast majority of the child’s genetic blueprint. This nuclear DNA is responsible for nearly all of a person’s traits, including their physical appearance, personality predispositions, and other inherited characteristics. Crucially, in this step, the defective mitochondrial DNA (mtDNA), which is located outside the nucleus in the cytoplasm of the egg, is left behind.
- Preparation of the Healthy Donor Egg: Simultaneously, an egg is obtained from a healthy woman who acts as a mitochondrial donor. This donor egg possesses healthy mitochondrial DNA. The key step here is to remove all of the donor egg’s nuclear DNA, leaving only its healthy mitochondria intact. This ensures that the resulting embryo will not inherit any of the donor’s nuclear traits.
- Pronuclear Transfer: This is the core of the “three-parent” technique. The genes (nuclear DNA) that were removed from the couple’s fertilized egg are then carefully injected into the enucleated fertilized egg from the healthy donor. This precise transfer is known as pronuclear transfer because it involves the transfer of the pronuclei (the nuclei of the sperm and egg before they fuse) from the affected couple’s fertilized egg into the donor’s enucleated egg.
- Embryo Development and Implantation: The resulting embryo now contains the nuclear DNA from the intended parents (the mother and father who wish to have the child) and the healthy mitochondrial DNA from the donor woman. This composite embryo can then develop in vitro. Once developed to an appropriate stage, it is implanted into the womb of the woman who wants to have a healthy, genetically related baby.
As a direct consequence of this procedure, the baby inherits all the DNA responsible for its main traits from its two intended parents (the nuclear DNA). Additionally, it receives a small amount of healthy mitochondrial DNA from the woman who donated the egg. This small contribution of healthy mtDNA is what leads to the informal, but widely used, term “three-parent” babies, as the child’s genetic makeup originates from three individuals: the mother, the father, and the mitochondrial donor. This method offers a revolutionary way to break the cycle of inherited mitochondrial diseases within families.
Early Results: Encouraging Progress and Expert Perspectives
The initial outcomes of the British study using the mitochondrial donation technique have been met with significant enthusiasm from the scientific and medical communities. The new study specifically involved babies born to seven women who faced a high risk of transmitting serious, disease-causing mitochondrial DNA mutations to their offspring.
The results, as reported by the researchers, indicate that the mother’s disease-causing mitochondrial DNA mutations were either undetectable or present at levels so low that they are unlikely to cause disease in the children. This is a monumental achievement, as it suggests the procedure successfully prevented the transmission of the debilitating genetic defects.
The human impact of this research is profound. As Doug Turnbull, a professor of neurology and a key member of the Newcastle University team that has been developing this technique for over a decade, states, “Mitochondrial disease can be devastating for the family. It can be tragic.” He passionately adds, “This is an important breakthrough — a big step forward.” His words underscore the immense relief and hope this technology offers to families who have endured generations of suffering from these often-fatal conditions.
The optimism is shared by other leading experts. Dietrich Egli, an associate professor of developmental cell biology at Columbia University, who has long advocated for lifting federal restrictions on similar research in the U.S., describes the work as “a landmark advance. It is pioneering work.” He further emphasizes, “It is extraordinary — no question about it.” Such strong endorsements from figures within the scientific establishment highlight the perceived significance and potential of this genetic technology.
Robin Lovell-Badge, a developmental biologist at the Francis Crick Institute in London, who authored an accompanying editorial to the published papers, eloquently articulates the procedure’s benefit: “A child with one of these conditions can be in a lot of pain, suffer all sorts of problems and die. It’s truly horrible to have to watch your child slowly die of something that bad.
It’s heartbreaking.” He concludes, “So for women at risk of having children with serious mitochondrial diseases, this provides them with an option to have children without suffering. It’s very encouraging.” The focus remains squarely on preventing profound suffering and offering families a viable pathway to healthy offspring.
Adding to the positive outlook, Professor Turnbull confirms the current well-being of the children, stating, “All the children are well and continue to meet their developmental milestones.” This crucial update provides tangible evidence of the procedure’s success in the short term. While the researchers acknowledge that the research is still in its relatively early stages and requires additional long-term follow-up and monitoring, the initial results offer a strong foundation for optimism about the future of this transformative genetic technology in reproductive medicine.
Ethical Concerns and Worries About Risks
Despite the scientific breakthroughs and the hope offered by mitochondrial donation, the technique is not without its critics. Significant ethical concerns and worries about potential risks temper the widespread enthusiasm. These concerns largely revolve around the safety of the procedure itself, its long-term implications, and the broader societal precedents it might set.
One primary area of concern is the risks to the babies themselves that may not yet be apparent. While the initial follow-up has shown healthy development, some critics argue that the long-term effects of altering a child’s genetic makeup, even a small portion like mitochondrial DNA, are unknown. They worry about unforeseen health issues that might emerge years or even decades later. This uncertainty fuels calls for extreme caution and extended monitoring periods.
Beyond the child, risks to the women involved are also highlighted. Francois Baylis, a distinguished research professor emerita at Dalhousie University in Canada, articulates concerns about both the women receiving the embryo and the women donating the eggs. For egg donors, there are worries about potential complications from the egg retrieval process, such as dangerous hyperstimulation of their ovaries, a known risk of fertility treatments. For the intended mothers receiving the embryo, there are standard risks associated with IVF procedures. Baylis emphasizes this uncertainty, stating, “We don’t know the future.”
A more profound ethical debate centers on the concept of “designer babies.” Critics like Stuart Newman, a professor of cell biology and anatomy at New York Medical College, vociferously voice these concerns. Newman calls the approach “dangerous,” asserting it is both “biologically dangerous” due to potential unforeseen effects on the human genome, and “dangerous culturally.”
He argues that by allowing genetic manipulation, even for disease prevention, society risks opening a “slippery slope” that could lead to “a full-fledged eugenics program.” In such a program, genes might be manipulated not just to prevent disease but to enhance traits, leading to the creation of “designer babies” based on parental preferences for appearance, intelligence, or other characteristics.
A significant point of contention is that these genetic changes can be passed down for generations. Critics argue that any mistakes or unintended consequences introduced through such manipulation could result in deleterious mutations being introduced into the human gene pool, with unknown and potentially irreversible long-term impacts.
Baylis further articulates a philosophical concern about the emphasis on having children with one’s “own genes.” She questions, “What you’re seeing is this sense that, ‘My genes are very valuable. My genes are the only ones worth reproducing.’ And I think that’s always worth questioning.” She suggests that this intense focus might overshadow other viable options for couples at risk of transmitting mitochondrial disorders, such as adoption, which she argues is a perfectly valid and loving way to build a family. These ethical and safety concerns underscore the complex societal implications of this cutting-edge genetic technology, inviting ongoing debate and careful consideration.
Global Regulatory Landscape and Future Outlook
The regulatory landscape surrounding mitochondrial donation is highly varied across the globe, reflecting the diverse ethical and scientific comfort levels with this groundbreaking technology. In the United States, current federal regulations explicitly prevent the procedure from being used to produce children. This strict stance reflects ongoing debates about safety, efficacy, and ethical implications. However, this hasn’t entirely halted the practice globally.
In 2016, a New York doctor controversially reported creating a “three-parent baby” for a Jordanian family in Mexico, highlighting the regulatory loopholes and the desire of some families to pursue the procedure despite prohibitions in their home countries.
Beyond the U.S., other nations have taken different approaches. Australia has notably legalized the procedure, signaling a progressive stance and a willingness to embrace the technology under regulated conditions. Similarly, doctors in some other countries, including Greece and Ukraine, have reportedly used the technique. Interestingly, their stated purpose in some cases has been to try and help infertile women have babies, even though scientific consensus generally indicates that the method’s effectiveness for addressing infertility is unclear and unproven. This highlights a potential misuse or misunderstanding of the technology’s primary intended purpose, which is to prevent mitochondrial disease transmission.
Critics like Francois Baylis express concern that the existence and limited legalization of such procedures could gradually “normalize the fact that it’s appropriate to take this material and to tinker with it.” She fears this could be perceived as a step toward “the pursuit of the perfect baby,” driven by subjective ideals. This sentiment underscores the “slippery slope” argument, where initial, well-intentioned applications for disease prevention might eventually lead to broader genetic manipulation for enhancement.
However, proponents of the technology offer a counter-argument regarding regulation and its distinct nature from other genetic interventions. Professor Doug Turnbull, from Newcastle University, acknowledges that the research is still at a relatively early stage, thus necessitating additional follow-up research and ongoing monitoring of the children.
Yet, he strongly asserts that reproductive technologies in Britain, where the study was conducted, and many other countries are “highly regulated.” He expresses confidence that “there are enough checks and balances in the system to prevent this from becoming a slippery slope to designer babies.” This viewpoint emphasizes the role of robust regulatory frameworks in controlling the application of such powerful technologies.
Furthermore, other experts stress the fundamental difference between mitochondrial donation and more controversial gene-editing techniques like CRISPR. Robin Lovell-Badge explicitly states, “This is totally different.” He clarifies that mitochondrial donation is purely about “avoiding a serious disease” by replacing defective mitochondria, without altering the nuclear DNA that defines a person’s core traits. He concludes,
“If you care about peoples’ health, peoples’ desire to have genetically related children, then I see no reason why you should not accept these methods.” This perspective frames the technology as a compassionate medical intervention designed to alleviate suffering, rather than a tool for genetic enhancement. The ongoing global discussion reflects the complex balance between scientific progress, ethical considerations, and the desire of families to have healthy children.